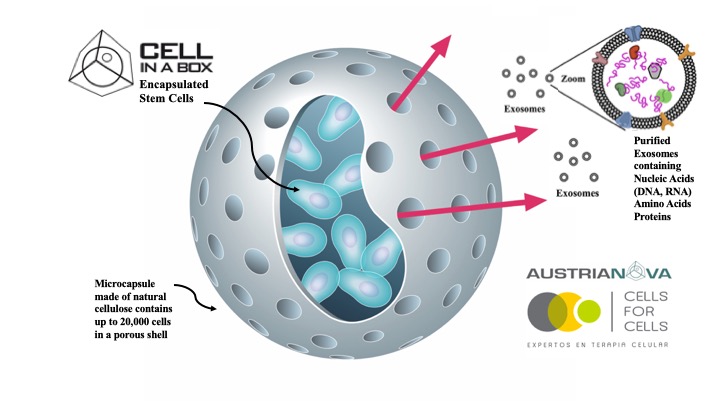
SINGAPORE, 2020-May-22 — /EPR BIOTECH NEWS/ — Austrianova and Cells for Cells have just jointly published a ground breaking, peer reviewed, scientific publication on a novel, cost and time-saving method to generate extra-cellular vesicles (EVs) from encapsulated Mesenchymal Stem Cells (MSCs). These EVs are known to mediate many of the therapeutic effects of stem cells. The authors show that Austrianova’s proprietary Cell-in-a-Box encapsulation technology can be used to produce and deliver EVs from encapsulated MSC’s, as demonstrated using Cells for Cells proprietary MSCs. The publication, which was co-authored with their academic partners, the University of the Andes, Chile and the University of Veterinary Medicine Vienna, Austria appeared in prestigious international journal “Frontiers in Pharmacology†(Front. Pharmacol., 21 May 2020 | https://doi.org/10.3389/fphar.2020.00679  https://www.frontiersin.org/articles/10.3389/fphar.2020.00679/full)
Currently, EVs have to be purified from cell culture conditioned media using tedious, costly and time-consuming protocols that are difficult to perform under Good Manufacturing Practices (GMP) conditions. The Cell-in-a-BoxÒ encapsulation technology allows efficient enrichment of EVs at high concentration since they are released from the encapsulated cells via the semipermeable pores, which selectively enable the release of small particles but not of the MSCs. Moreover, Cell-in-a-BoxÒ provides 3D culture conditions for the MSCs. The technology can be used in cell culture allowing GMP production. Alternatively, the encapsulated cells can be implanted into patients as a retrievable delivery device that shields the cells from clearance, whilst they continuously produce EVs, growth factors, hormones and other small therapeutically relevant molecules. Moreover, the EVs produced after encapsulation can themselves be used as drug-loaded delivery vehicles. This technology will be invaluable for the treatment of regenerative diseases and Inflammatory disease.
Maroun Khoury, CSO of Cells for Cells said “this is a multifaceted project bringing together different expertise  to support the burgeoning field of EV-based therapies. It will be interesting to test in the near future, the continuous release of EVs in a in vivo context. At a personal level, it was a great way to stay connected with colleagues that I met while living in Singapore â€.
Brian Salmons, CEO of Austrianova said “we are pleased that these results representing the culmination of a long term project with our colleagues at Cells for Cells are finally publicly available. The encapsulation of stem cells as a means to produce exosomes using our Cell-in-a-BoxÃ’ is an exciting technological breakthrough that is applicable for all stem cell types.â€
About Austrianova
Austrianova, part of the SG Austria Group, is a biotech company with a global footprint and headquarters in Singapore. Austrianova utilizes a novel and proprietary technology for the encapsulation of living mammalian (Cell-in-a-Box®) and bacterial (Bac-in-a-Box®) cells. Cell-in-a-Box® protects the encapsulated cells from rejection by the immune system, allows cells to be easily transported, stored and implanted at specific sites in patients. The technology, which has been proven safe and efficacious in clinical trials carried out in Europe, allows companies to develop any kind of cells as a one-for-all living pharmaceutical. Bac-in-a-Box® is a similar protective device adapted for encapsulation of probiotic bacteria where it has human food and animal feed applications due to its ability to extend storage under lyophilized conditions and to protect encapsulated bacteria against destruction by stomach acid. Austrianova now also offers GMP4Cells that includes competitively priced Master Cell Bank and Working Cell Bank production as well as “Fill and Finish†services for cell therapy products (such as stem cell therapies, biologics produced from cells e.g. vaccines, antibodies, recombinant proteins etc).
About Cells for Cells
Cells for Cells is a Chilean biotechnological company dedicated to the research, development and commercialization of innovative cellular therapies, complying with high standards of scientific, technological and international quality, through manufacturing processes certified under ISO 9001: 2015. Each therapy is produced in our labs with GMP standards, being the first biotech company, with such high-quality standards at Latin American level. Our therapies are applied by duly certified specialists.
Logos:

