CAMBRIDGE, 25-Sep-2024 — /EuropaWire/ — In recent years, brain-computer interface (BCI) technologies have garnered widespread attention with the promise of revolutionizing how humans interact with machines—potentially replacing traditional interfaces like touch screens and mice, and offering breakthroughs in areas like speech decoding, wheelchair control, and even driving cars. Although much of the public focus has been on Elon Musk’s Neuralink, new research from market intelligence firm IDTechEx highlights that the BCI market extends far beyond this one company.
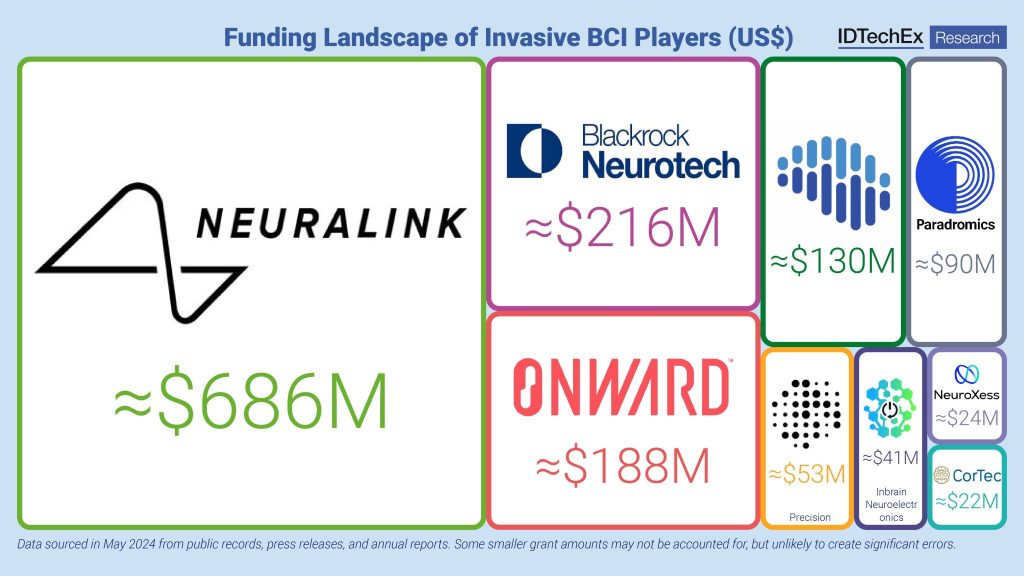
IDTechEx’s latest report reveals that while Neuralink is a prominent player, the market is also being shaped by a number of other invasive BCI developers, as well as a well-established ecosystem of non-invasive solution providers. Several companies that predate Neuralink—such as Blackrock Neurotech, Synchron, Onward Medical, and Paradromics—have also been leading the charge in this competitive landscape. These companies have collectively attracted close to $1.5 billion in funding as they work to address some of the key challenges facing neural interfaces.
While most players are focused on solving similar technical hurdles, their approaches differ. Neuralink, for instance, is tackling the need for specialist surgeons by developing a robotic system capable of implanting ultra-thin electrodes into the brain. In contrast, Blackrock is advancing its well-established Utah array to create more flexible and high-channel-count products. The companies also diverge in their emphasis on wireless solutions.
Other competitors are exploring less invasive options. Onward Medical is focusing on electrocorticography (ECoG), which places sensors within the skull but only on the brain’s surface. Meanwhile, Synchron uses a stent-like device that interacts with the inside of blood vessels.
Over the coming years, the BCI market will see a significant increase in clinical trial activity as these various technologies are tested in real-world settings. Companies will need to demonstrate tangible improvements in quality of life for people who rely on assistive technologies for communication and mobility in order to maintain investor confidence and win broader regulatory support.
While invasive BCIs receive much of the attention, non-invasive solutions remain crucial. For decades, electroencephalography (EEG) caps have been used in neuroscience research to capture brain signals, with applications ranging from wheelchair control to speech decoding. IDTechEx believes the next few years will be critical in determining whether non-invasive BCIs will break into consumer markets or remain confined to research and emotional monitoring applications.
Looking ahead, IDTechEx forecasts growth for both invasive and non-invasive BCI technologies, with the overall market expected to surpass $1.6 billion by 2045. Non-invasive solutions are likely to gain traction first, followed by the commercialization of invasive technologies from players like Neuralink. However, the long-term potential in the assistive technology market may well belong to companies able to provide successful, scalable solutions.
For a deeper dive into the global BCI landscape, including market forecasts over the next two decades, the full IDTechEx report, “Brain Computer Interfaces 2025-2045: Technologies, Players, Forecasts,” can be accessed at www.IDTechEx.com/BCI.
SOURCE: EuropaWire











 Gum Health
Gum Health